Benjamin B. Land, PhD


Dr. Ben Land is surprised to find himself in cannabinoid neurobiology because he started his career as a behaviorist, observing honeybee dances. While working in the UW lab of Dr. Charles Chavkin, Ben discovered his desire to affect people tangibly by exploring the neurobiology of compounds such as opioids.
During his career Ben has studied the role of opioids in feeding behavior, anxiety, stress, addiction, and dysphoria, using animal models. He also has explored the differences in opioid receptor activation between males and females.
He then became fascinated by the intersection of opioids and cannabinoids. We know cannabinoids work--they synergize well with opioids for pain management--but we don't yet know which receptors in the brain they bind to.

Ben's current research path is governed by his two grant applications currently under review at NIH.
In an R01 grant application Ben proposes to study the synergy between terpenoid compounds and CBD. Cannabis has hundreds of terpenoid compounds, many of which it shares with other plants such as lemons and pines. Terpenoids have been shown to provide pain relief and lessen stress. Ben will implement "behavioral, molecular, and imaging techniques to determine if combinations of cannabidiol and terpenoid compounds are effective in treating pain."
For an R21 grant, Ben proposes to use neurobiological methods to study the intersection of cannabinoids and opioids in the context of pain and addiction. Can CBD lessen the need for opioid use?
Ben describes neurobiology as the "bridge" between scientific techniques and human health. By knowing the mechanisms of cannabis' effects on humans we can develop compounds that target issues--stress, pain, itching--but can eliminate the side effects.
In addition to his purely scientific endeavor, Ben also aspires to legitimize the study of CBD. While scientists and grateful patients know CBD is effective, papers describing CBD research are often overlooked by the front line journals. Hypothesis-driven neurobiological research on CBD can help establish value in the scientific community.
Current Funding as PI
SCAN Design Foundation (Land, PI); 2020-22; $50k
Title: Targeting reactive oxygen species (ROS) to enhance opiate analgesic action. Summary: The goal of this grant is to understand how ROS play a role in opioid tolerance, and to develop potential treatments using ROS inhibitors.
NIH (NINDS) R01 (Dhaka, PI, Primary role, co-investigator); 2020-25; $250k/year
Title: Identifying new targets in pain, utilizing the novel analgesic AS1. Summary: This project will characterize a novel compound (AS1) in a variety of models of pain and reward/aversion behavior.
NIH (NIDA) P30 Pilot Project (Chavkin, PI, Project PI Land); 2021-22 $50k
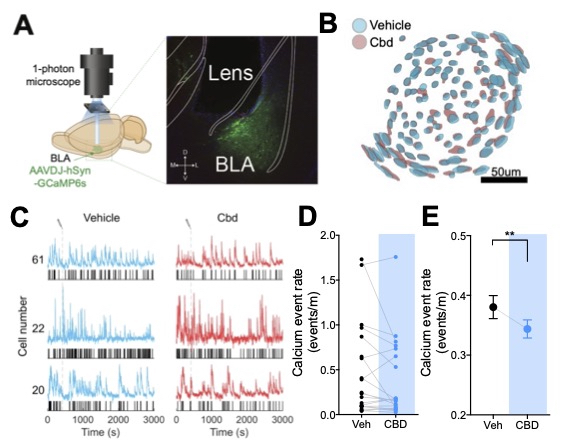
Summary: This work uses 2-photon imaging and spatial light modulation to determine whether cannabidiol modulates amygdala activity in pain states.
NIH (NCCIH) R01 (Land, PI), Pending
Title: Cannabidiol and terpenoid interactions in amygdalar regulation of pain states. Summary: This work implements behavioral, molecular, and imaging techniques to determine if combinations of cannabidiol and terpenoid compounds are effective in treating pain, with a focus on the amygdala.
Selected Publications
Abraham AD, Casello SM, Schattauer SS, Wong BA, Mizuno GO, Mahe K, Tian L, Land BB, Chavkin C. Release of endogenous dynorphin opioids in the prefrontal cortex disrupts cognition. Neuropsychopharmacology. 2021 Dec;46(13):2330-2339. doi: 10.1038/s41386-021-01168-2. Epub 2021 Sep 20. PMID: 34545197; PMCID: PMC8580977.
Abraham AD, Leung EJY, Wong BA, Rivera ZMG, Kruse LC, Clark JJ, Land BB. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology. 2020 Jun;45(7):1105-1114. doi: 10.1038/s41386-019-0585-3. Epub 2019 Dec 7. PMID: 31812152; PMCID: PMC7235274.
Schattauer SS, Bedini A, Summers F, Reilly-Treat A, Andrews MM, Land BB, Chavkin C. Reactive oxygen species (ROS) generation is stimulated by κ opioid receptor activation through phosphorylated c-Jun N-terminal kinase and inhibited by p38 mitogen-activated protein kinase (MAPK) activation. J Biol Chem. 2019 Nov 8;294(45):16884-16896. doi: 10.1074/jbc.RA119.009592. Epub 2019 Oct 1. PMID: 31575661; PMCID: PMC6851317.
Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, Johnson SD, Land BB, Chavkin C. Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J Neurosci. 2018 Sep 12;38(37):8031-8043. doi: 10.1523/JNEUROSCI.0653-18.2018. Epub 2018 Aug 3. PMID: 30076211; PMCID: PMC6136151.
Schattauer SS, Land BB, Reichard KL, Abraham AD, Burgeno LM, Kuhar JR, Phillips PEM, Ong SE, Chavkin C. Peroxiredoxin 6 mediates Gαi protein-coupled receptor inactivation by cJun kinase. Nat Commun. 2017 Sep 29;8(1):743. doi: 10.1038/s41467-017-00791-2. PMID: 28963507; PMCID: PMC5622097.
Interview by Nancy Ottman Press, 1/26/22





